surface energy test water drop|surface energy of solids study : distributing A contact angle gives us an indication of how well (or how poorly) a liquid will spread over a surface. While formulating an ink, contact angles provide a useful indicator of how a modification to the ink will affect its spreading.
Fotos de las acompañantes. Modelos profesionales. Trato d.
{plog:ftitle_list}
Resultado da PAY INTELIGÊNCIA FINANCEIRA E FOMENTO COMECIAL LTDA. Pay, como interveniente anuente e a empresa ré, como contratada. A .
surface free energy methods
In the case of using water, the optimal volume of the drops would be between 10 and 20 µL to allow the action of both the surface tension and the gravitational force determine .
Surface free energy measurement with an optical tensiometer. The most common way to measure surface free energy is through sessile drop measurements with the optical tensiometer. Depending on the surface free . To be sure you're getting the right coating for your application consider employing a water drop contact angle measurement tool to assess the surface energy, attraction and .Measuring the surface energy of a liquid is simple and straightforward. The surface energy of a liquid is identical to its surface tension, and a variety of techniques exist to measure liquid .
high moisture meter reading
surface energy of solids study
The main application of contact angle measurement is determination of the wetting behavior of a solid surface with a specific liquid. Water drops are used to measure hydrophobicity or .
A contact angle gives us an indication of how well (or how poorly) a liquid will spread over a surface. While formulating an ink, contact angles provide a useful indicator of how a modification to the ink will affect its spreading.In materials science, the sessile drop technique is a method used for the characterization of solid surface energies, and in some cases, aspects of liquid surface energies. [1] .Surface energy comes into play in wetting phenomena. To examine this, consider a drop of liquid on a solid substrate. If the surface energy of the substrate changes upon the addition of the . The formula for surface energy is: γ= W A γ = W A. Where: – γ is the surface energy. – W is the work or energy required to create a new surface. – A is the area of the .
Sessile-drop goniometry. The method of measuring contact angles in the protocol is called sessile-drop goniometry. It is performed by recording a video of a water drop on a solid surface and .
Molecules near the surface have a high potential energy. As many of them fall beneath the surface as the surface area is decreased, this potential energy is converted to kinetic energy. Conversely, if a spherical .An illustration of the sessile drop technique with a liquid droplet partially wetting a solid substrate. θ C is the contact angle, and γ SG, γ LG, γ SL represent the solid–gas, gas–liquid, and liquid–solid interfaces, respectively.. In materials science, the sessile drop technique is a method used for the characterization of solid surface energies, and in some cases, aspects of .A powerful instrument for quality control of pretreated, coated, or cleaned surfaces: The MSA One-Click SFE Mobile Surface Analyzer measures wettability by means of the surface free energy automatically and extremely fast.. With just one click, the MSA doses parallel drops of two liquids, followed by the direct analysis of the contact angles and the derived results of the .
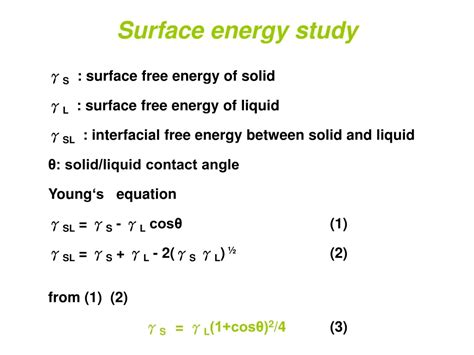
Schematic of a liquid drop showing the quantities in the Young equation. The contact angle (symbol θ C) is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interface at their intersection. It quantifies the wettability of a solid .This can occur with a high surface-energy solid (such as a metal), or a low surface-tension liquid. If γ sv < γ ls, then cos θ will be negative, and θ is therefore > 90 o (and the droplet dewets). This can occur with a low surface-energy solid, or a high surface-tension liquid (such as water).a pendant shape. The formation of this drop is explained by the fact that the surface tension forces cancel out the gravitational force acting on the drop. D ethanol water D E D E D S Figure 2: Typical ethanol and water pendant drops. Now that you have learnt about surface tension, you are likely to realise how it causes many phenomena that we .
high rated moisture meter for wood
Controlling unequal surface energy results caused by test liquids: the case of UV/O3 Treated PET . Lee, S., Pradel, K. C. & Wang, Z. L. Harvesting water drop energy by a sequential contact .
Smithers use a PG-3 Pocket Goniometer not only to measure the static contact angle, but also the volume and spread of a drop of liquid on the surface over time. This dynamic absorption test (DAT) is applied to test the liquid absorbency of paper or board. DAT can be used to determine the degree of sizing or water hold out of paper with time.
8000 identical water drops are combined to form a big drop then the ratio of the final surface energy to the initial surface energy of all the drops t asked May 20, 2020 in Physics by Subodhsharma ( 86.7k points)
Surface free energy is most typically measured through sessile drop measurements but the force tensiometer can be used as well. Surface free energy measurement with an optical tensiometer. The most common way to measure surface free energy is through sessile drop measurements with the optical tensiometer.In a sessile drop contact angle measurement, a drop of liquid (typically water) is placed on the surface of the sample of interest and the contact angle is measured without moving the droplet. Calculating the contact angle between the droplet and surface using Young’s equation necessitates several assumptions about the surface, as it only .A drop striking a liquid surface; in this case, both the drop and the surface are water. In fluid dynamics, drop impact occurs when a drop of liquid strikes a solid or liquid surface. The resulting outcome depends on the properties of the drop, the surface, and the surrounding fluid, which is most commonly a gas.Most commonly, surface energy is measured with water. When a droplet of water is placed on a surface, it will bead up to some extent. Theoretically, if the surface energy is zero, the droplet will be a perfect sphere. Conversely, if the surface energy is infinitely high, the droplet will form a perfectly uniform film.
B.-Petermann, 2003 (139) Contact angle γ s = 33.1 mJ/m 2 (γ s d = 32.3; γ s p = 0.8); Test liquids: water, diiodomethane, and formamide, measured 20 oC by sessile drop method.Roll-coated polymer topcoat applied to carbon steel; surface degreased with ethanol, cleaned with detergent, and rinsed in distilled water.With the help of the Drop Shape Analyser Kruss DSA 10, contact angles of the two test liquids water and diiodomethane (CH 2 I 2) can be measured to determine the surface energy to be around 36 mN/m (see fig. 3). After .
Dyne inks operate under the principle of surface energy or wetting, a phenomenon that correlates to the potential adhesive ability of a surface. When a substance comes in contact with a new material, the substance can show .Wettability. Djebbar Tiab, Erle C. Donaldson, in Petrophysics (Third Edition), 2012. Sessile Drop Measurement of Contact Angles. The sessile drop method is often used to make direct measurements of the contact angle to determine preferential wetting of a given solid by oil and water. A smooth, homogeneous surface is necessary for this test; a polished quartz surface .A guide to the meaning of surface energy, how it can be calculated using contact angle measurements and models, and how it can be tuned with surface treatment. . Electrical Test Boards Four-Point Probe LED Measurement System Potentiostat Solar Cell I-V Test System Source Measure Unit Solar Simulators Solar Simulator Solar Cell Testing Kit .
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged.. At liquid–air interfaces, surface tension results from the greater attraction of . Figure S1 shows a comparison of a static water drop without and with the presence of a ring drop holder on an OTS-modified silicon wafer. Figure S2 shows SEM images of the textured SH_20_20 and SH 20_30 surfaces. Figure S3 shows a plot of friction data between a 20 μL water drop and an OTS-modified hydrophobic silicon surface as a function of .Surface free energy (SFE) is the work that would be necessary to increase the surface area of a solid phase. SFE has a decisive influence on the wettability of solids by liquids. It is therefore an important parameter for the optimization of coating processes, but .If a solid surface with high surface energy comes into contact with a water drop, a low contact angle forms. This means that the water drops spread on the surface. Glass, ceramics and many metals are examples of solids whose surfaces have a high surface energy naturally. A low surface energy indicates poor wetting and thus poorer adhesion.
If \(E _1 \) be the total surface energy of 1000 small drops of water and \(E_2\) be the surface energy of single big drop of water, the \( E_1 : E_2\) is x : 1 where x = ___. jee main 2024; Share It On Facebook Twitter Email Play Quiz Games with your School Friends. . Find MCQs & Mock Test. JEE Main 2025 Test Series; NEET Test Series; Class . Figure 2.10a shows the drop profile of a 5 mg water drop on a superhydrophobic surface with a measured contact angle of 156° using ellipse fitting . After 40 min exposure in air, the drop volume reduces to 0.3 μL and drop distortion (by gravity) is visibly reduced. The contact angle is calculated to be 173° by ellipse fitting.
When translated into energy per unit area, the surface energy of metals and inorganic salts is usually in the range of 1-2 J/m 2. Example: The sublimation energy of bulk gold is 334 kJ/mol, and the surface energy is 1.5 J/m 2. What percentage of the bulk bonding energy is lost by atoms at the (111) surface of a gold crystal?
The adhesion of water droplets to leaves is important in controlling rainfall interception, and affects a variety of hydrological processes. Leaf water drop adhesion (hereinafter, adhesion) depends not only on droplet formulation and parameters but also on the physical (leaf roughness) and physico-chemical (surface free energy, its components, and .

surface energy of a droplet
webYou can always find a new game to play with the wide variety of game types available, such as Action, Multiplayer, and Shooting games. Grab your device and have fun! Best free .
surface energy test water drop|surface energy of solids study